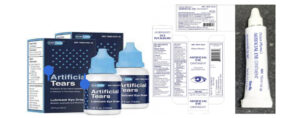
The Government Analyst- Food and Drug Department (GA-FDD) hereby alerts the general public of a voluntary recall of EzriCareArtificial Tears, Delsam Artificial Tears and Delsam Artificial Eye Ointment (Batch No. H29), manufactured by Global Pharma Health in India due to possible bacterial contamination.
The recalled products are being investigated for possible contamination with a rare, extensively drug-resistant strain of Pseudomonas aeruginosa. The use of contaminated artificial tears increases risk of eye infections that could result in blindness or death. Associated adverse events include yellow, green or clear discharge from the eye, discomfort or pain, redness of the eye or eyelid, blurry vision, and increased sensitivity to light.
Artificial Tears Lubricant Eye Drops, 10mg in 1ml, ½ fl oz (15ml) are used as an eye protectant against further irritation or to relieve dryness of the eye for the temporary relief of discomfort due to minor irritations of the eye, or exposure to wind or sun. Artificial Eye Ointment is used as an eye lubricant and to relieve dryness of the eyes. The recalled products were distributed nationwide in the United States of America (USA) over the internet. Consumers are therefore being alerted for possible direct purchases via internet.
Based on investigations conducted, the EzriCare Artificial Tears, Delsam Artificial Tears and Delsam Artificial Eye Ointment are not registered by the GA-FDD and have not been seen to date on the local market.
Consumers are however urged to be on the lookout for the implicated products and those who have the recalled product(s)should immediately stop use, discard appropriately and/or make contact with the GA-FDD on telephone numbers 222-8857/60 or WhatsApp on 222-8011 for further information on reporting of adverse event(s).
-END–
Address: IAST Building, University of Guyana Compound, Turkeyen, Georgetown, Guyana , P.O. BOX 1019
Telephone: 592-222-8856-60 website: gafdd.gy Email: gafddgy@gmail.com Facebook Page: Government Analyst Food and Drug Department